REMOVAL OF IONS OF Al3+ AND Co2+ PHOSPHORUS-CONTAINING PRODUCT FROM THE BRAN
DOI:
https://doi.org/10.14258/jcprm.2018043761Keywords:
rice bran, aluminum ions, cobalt ions, removalAbstract
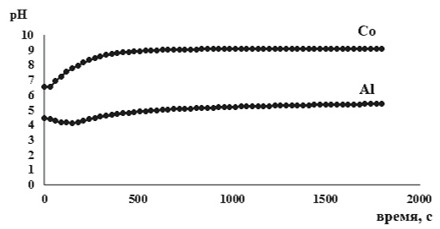
The process of removal aluminum and cobalt ions from aqueous solutions with a phosphorus-containing product containing inositol hexaphosphoric acid derivatives obtained from rice production wastes of Far Eastern selection varieties – rice flour, and optimal conditions were determined. It is established that the efficiency of removal of metal cations by the phosphorus-containing product depends not only on their nature, but also on the initial concentration of ions in solutions. The degree of extraction of aluminum ions reaches 99%, and cobalt – 76%. The principal possibility of using phosphorus-containing products of plant origin in the development of environmentally safe materials for the removal of metal ions in technologies requiring the use of green chemistry methodology is proposed.
Downloads
References
2. SanPiN 2.1.4.1074-01. Pit'yevaya voda. Gigiyenicheskiye trebovaniya k kachestvu vody tsentralizovannykh sistem pit'yevogo vodosnabzheniya. Kontrol' kachestva. 01.01.2002. [Sanitary rules and regulations 2.1.4.1074-01. Drinking water. Hygienic requirements for water quality of centralized drinking water supply systems. Quality control. 01/01/2002]. 53 p. (in Russ.).
3. Ob utverzhdenii normativov kachestva vody vodnykh ob"yektov rybokhozyaystvennogo znacheniya, v tom chisle nor-mativov predel'no dopustimykh kontsentratsiy vrednykh veshchestv v vodakh vodnykh ob"yektov rybokhozyaystvennogo znacheniya: prikaz Federal'nogo agentstva po rybolovstvu ot 18.01.2010. [On approval of water quality standards for water bodies of fisheries significance, including standards for maximum permissible concentrations of harmful substances in waters of water bodies of fisheries significance: the order of the Federal Agency for Fishery dated January 18, 2010]. N20, 09.02.2010, 153 p. (in Russ.).
4. Gigiyenicheskiye normativy GN 2.1.5.1315-03. Predel'no dopustimyye kontsentratsii khimicheskikh veshchestv v vode vodnykh ob"yektov khozyaystvenno-pit'yevogo i kul'turno-bytovogo vodopol'zovaniya. [Hygienic standards GN 2.1.5.1315-03. Maximum permissible concentrations of chemicals in the water of water bodies of drinking and household water use]. Moscow, 2003, 94 p. (in Russ.).
5. Rakhmanin YU.A., Mikhaylova R.I., Sysina A.N. Chistaya voda: Sbornik materialov Mezhregional'nogo kon-gressa. [Clear water: Collection of materials of the Interregional Congress]. Perm, pp. 10–21. (in Russ.).
6. Rossiyskiy statisticheskiy yezhegodnik. [Russian statistical yearbook]. Moscow, 2017, 686 p. (in Russ.).
7. Zemnukhova L.A., Panasenko A.E., Artem'yanov A.P., Tsoy E.A. BioResources, 2017, vol. 10, no. 2, pp. 3713–3723. DOI: 10.15376/biores.10.2.3713-3723.
8. Zemnukhova L.A., Makarenko N.V., Tishchenko L.Ya., Kovaleva E.V. Russian Journal of Bioorganic Chemistry, 2010, vol. 36, no. 7, pp. 941–943. DOI: 10.1134/S1068162010070241.
9. Zemnukhova L.A., Tomshich S.V., Mamontova V.A., Komandorova N.A., Fedorishcheva G.A., Sergiyenko V.I. Zhurnal prikladnoy khimii, 2004, vol. 77, no. 11, pp. 1901–1904. (in Russ.).
10. Zemnukhova L.A., Isay S.V., Shkorina Ye.D., Busarova N.G. Zhurnal prikladnoy khimii, 2006, vol. 79, pp. 1554–1557. (in Russ.).
11. Saburov K.A., Kamilov Kh.M. Chemistry of Natural Compounds, 1989, vol. 25, no. 6, pp. 695–698. DOI: 10.1007/BF00598269.
12. Barrientos L.G., Murthy P.P.N. Carbohydrate Research, 1996, vol. 296, pp. 39–54. DOI: 10.1016/S0008-6215(96)00250-9.
13. Raboy V. Phytochemistry, 2003, vol. 64, no. 6, pp. 1033–1043. DOI: 10.1016/S0031-9422(03)00446-1.
14. Greiner R., Konietzny U. Food Technology and Biotechnology, 2006, vol. 44, no. 2, pp. 125–140.
15. Sergiyenko V.I., Zemnukhova L.A., Yegorov A.G., Shkorina Ye.D., Vasilyuk N.S. Rossiyskiy khimicheskiy zhurnal, 2004, vol. XLVIII, no. 3, pp. 116–124.
16. Iemma F., Cirillo G., Spizzirri U. et al. European Polymer Journal, 2008, vol. 44, no. 4, pp.1183–1190. DOI: 10.1016/j.eurpolymj.2008.01.024.
17. Li R., Liu L., Yang F. Journal of Hazardous Materials, 2014, vol. 280, pp. 20–30. DOI: 10.1016/j.jhazmat.2014.07.052
18. Kiss T., Zatta P., Corain B. Coordination Chemistry Reviews, 1996, vol. 149, pp. 329–346. DOI: 10.1016/S0010-8545(96)90036-3.
19. Martin C.J. Journal of Inorganic Biochemistry, 1995, vol. 58, pp. 89–107. DOI: 10.1016/0162-0134(94)00038-C
20. Bebort-Brigaud A., Dange C., Fauconnier N., Gerard C. Journal of Inorganic Biochemistry, 1999, vol. 75, no. 1, pp. 71–78. DOI: 10.1016/S0162-0134(99)00041-0.
21. Vasca E., Materazzi S., CarusoT. et al. Analytical and Bioanalytical Chemistry, 2002, vol. 374, no. 1, pp. 173–178. DOI: 10.1007/s00216-002-1469-6.
22. Makarenko N.V., Yarusova S.B., Azarova Yu.A., Zemnukhova L.A. Bulletin of the Far Eastern Branch of the Russian Academy of Sciences, 2015, no. 4, pp. 94–99. (in Russ.).
23. 23. Kolzunova L.G., Zemnukhova L.A., Fedorishcheva G.A., Kurylenko L.N., Sergienko V.I. Journal of Applied Chemistry, 2000, vol. 73, no. 10, pp. 1644–1651. (in Russ.).
24. DR 2700™ Portable Spectrophotometer. Methods/Procedures. Hach Company URL: http://www.hach.com/dr-2700-portable-spectrophotometer-with-lithium-ion-battery/product-downloads?id=7640439008&callback=bc
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors, which are published in this journal, agree to the following conditions:
1. Authors retain the copyright to the work and transfer to the journal the right of the first publication along with the work, at the same time licensing it under the terms of the Creative Commons Attribution License, which allows others to distribute this work with the obligatory indication of the authorship of this work and a link to the original publication in this journal .
2. The authors retain the right to enter into separate, additional contractual agreements for the non-exclusive distribution of the version of the work published by this journal (for example, to place it in the university depository or to publish it in a book), with reference to the original publication in this journal.
3. Authors are allowed to post their work on the Internet (for example, in a university repository or on their personal website) before and during the review process of this journal, as this may lead to a productive discussion, as well as more links to this published work.

